I worked on gene drives for a number of years jointly as a member of George Church and Flaminia Catteruccia’s labs at Harvard. Most of my effort was spent primarily on an idea for an evolutionary stable gene drive, which didn’t work but we learned some stuff, and I developed a sense for the real challenges with gene drives. It’s something that comes up often when I meet new people in the bio community, so this is my attempt at laying out my perspective. I will be upfront and say I haven’t worked in this field since 2019, and though I’ve tried to update my understanding based on 2024 literature, I might have things wrong based on the bleeding edge. If you disagree, please let me know on twitter or via email.
Ultimately this piece is a vibe on my part and is not a reflection on the official stances of anyone I’ve worked with! A few skilled scientists have pushed back on my pessimism with reasonable counterpoints, the most notable of them being Merrick Pierson Smela, but I hope by the end of the article you can understand why I think the technical details of gene drives are likely harder to overcome than we realize based on the history of evolutionary dynamics in mosquitoes.
you can find the TL;DR at the bottom of the post.
Transgenic GFP-expressing Anopheles mosquitoes (Dong et al)
Why gene drives would be awesome
Figure 1. Malaria-endemic regions throughout history (Source).
Mosquitoes are extremely dangerous. Maybe you learned this at a trivia night with friends, or by reading the news, or from reading a blog post, but it’s something that bears repeating – mosquitoes are extremely dangerous. They are likely the largest driver of death and human disease in recorded history, estimated to have caused upwards of 52 billion deaths globally, because of their propensity to carry viruses and parasites that make humans very ill. Even today they account for over half a million deaths from malaria globally annually, with another 250 million contracting the disease. When accounting for the full spectrum of vector-borne diseases, nearly 4 billion people live in areas where they are at risk of contracting dengue fever. Though we mostly think of mosquitoes as a harbinger of plagues that affect only the developing world, superpowers like America were home to diseases like malaria and dengue fever no less than 80 years ago, when we decided to undertake massive eradication efforts that are out of fashion in the modern world. Mosquitoes are a problem for humans, and have been for much longer than recorded history. Something rightfully needs to be done about them, but what?
Enter gene drives.
Some background on mosquitoes and gene drives
Figure 2. Life cycle of a mosquito. Thanks, CDC.
The idea of editing mosquitoes to block their vector transmission capabilities has been around for quite a while, dating back to the mid 20th century. Scientists, primarily Christopher Curtis, proposed the idea of driving selfish, anti-pathogenic genes into wild populations of mosquitoes with the hope of rendering them inert. Much fascinating and important work has been done exploring that topic by a number of incredible scientists like Austin Burt, but the idea of editing mosquitoes achieved renewed vigor in 2014 when my former colleagues Andie Smidler, Kevin Esvelt, Flaminia Catteruccia and George Church proposed the concept of using CRISPR/Cas9 systems to create evolutionarily stable gene drives. Before we talk about gene drives, it’s worth visiting some basics about mosquitoes that are underappreciated but highly relevant for a successful gene drive effort. I will focus mostly on the adult mosquito, though mosquitoes go through many stages, and it’s worth noting a relatively small (1-5%) percent of mosquitoes make it from eggs to adults (Figure 2).
Figure 3. Mating swarm showcasing nighttime meetup of thousands of mosquitoes hoping for their one shot at ensuring their lasting legacy through reproduction (Source: William Robert Shaw, HHMI Staff Scientist at Harvard University)
There are over 3000 species of mosquito. Gene drives work by using nucleotide-specific guide RNAs to create desired edits in mosquitoes. There is enough variability between mosquito species such that 1 SNP can render a drive construct broken. This means that a single identical gene drive construct will not work for every mosquito species.
Female mosquitoes mate only one time in their entire life. Once a female mosquito reaches maturity, they will engage in swarm mating (Figure 3), where they select one male mate who acts as their lifelong sperm donor. This male can go on to mate with other females, but the female is set with that male’s sperm for life – all of her progeny will be the result of his sperm. One edited male mosquito mating with one female can result in thousands of off-spring carrying (gene-drive) mutations. Particularly useful for population suppression (discussed more later).
Female mosquitoes carry diseases. Blood meals are taken only by female mosquitoes in order to develop eggs, and are taken by a female when some internal biological trigger signals that it’s time to produce more offspring. The blood acts as an important nutrient for the development of the eggs – no blood, no eggs. If we know that humans get diseases like malaria or Zika virus from mosquitoes, and only female mosquitoes actually bite humans, then we can now recognize that female mosquitoes are the dangerous ones.
Mosquitoes otherwise subsist mostly on sugar water. They don’t actually use the blood as a food source for themselves.
On to gene drives.
Gene drives allow for super-Mendelian inheritance of traits
Figure 4. Under normal circumstances, traits for an imaginary gene “A” are inherited following the standard 25-50-25 ratio pattern if both parents are carriers. If only 1 is a carrier and the other is dominant for AA, then 0% off-spring will have the “aa” phenotype.
At their core, gene drives are a clever genetic trick that forces a particular gene to be inherited by nearly all offspring, rather than the usual 50-50 chance in normal inheritance (Figure 4), to achieve super-Mendelian inheritance. Imagine a typical gene: when organisms reproduce, their offspring have a 50% chance of inheriting any particular version of that gene from either parent. Gene drives break this rule.
Figure 5. Gene drives can propagate a desired mutation through an entire population theoretically with only a few mating events. (Source)
The modern CRISPR-based gene drive works like a genetic copy machine. It contains two key components: the CRISPR machinery (Cas9) that can cut DNA, and a guide RNA that tells it exactly where to cut. When a mosquito carrying a gene drive mates with a wild mosquito, something fascinating happens in their offspring. The drive locates the natural version of its target gene on the chromosome inherited from the wild parent, cuts it, and forces the cell to repair the cut by copying over the drive-containing version. This only happens because the gRNA is specific to the gene we want to excise.
This means that instead of 50% of offspring inheriting the gene drive, nearly 100% do. Each generation, the number of mosquitoes carrying the drive doubles compared to normal inheritance. This exponential spread is what makes gene drives so powerful - and so concerning to many scientists.
Figure 6a and 6b. (Left, 6a) Allelic spread through a mosquito population where the goal is population replacement. This is done when you want to propagate a cargo through a population of mosquitoes without changing the number of mosquitoes. (Right, 6b) Dynamics of a population suppression drive, whereby either sterile males or sterile females released in an area suppress the total number of mosquitoes in a given area. (Figure source is from Catteruccia lab).
The drive can carry cargo along with it - genes that could, for instance, prevent mosquitoes from transmitting malaria or cause them to produce only male offspring. This is where the real power of gene drives for disease control comes in. In theory, you could release a small number of engineered mosquitoes and have the desired trait spread through the entire population within a few generations. Cargo selection matters in this case, but I mostly won’t talk about that in this piece. Just know that there are a number of groups working on this – their ideas range quite a bit, but some of my favorites include generation of anti-malarial peptides or porting over cytoplasmic incompatibility genes from Wolbachia-infected mosquito populations (Figure 6a). Some models, including one I’ve worked on, are towards population suppression drives whereby the goal is to nuke the population of mosquitoes in an area by creating sterile males. Because female mosquitoes only mate one time, a mating event between a sterile male and a wild-type female will result in a female mosquito being unable to ever produce viable offspring, which, when done at scale, can severely bottleneck a population – something that is good for vector control (Figure 6b). There are issues with that approach, because some scientists argue mosquitoes play an important role in various ecosystems, but these are not the core problems I want to address in this piece. What is worth noting is that continuous releases, generally seasonally, of sterile males or females is required to maintain the suppression of the population. Since we can’t drop the population to 0 entirely, an injection is required to keep up the effect. If you want to talk more about the tradeoffs of each approach, you can email me.
Endonuclease action, decomposed
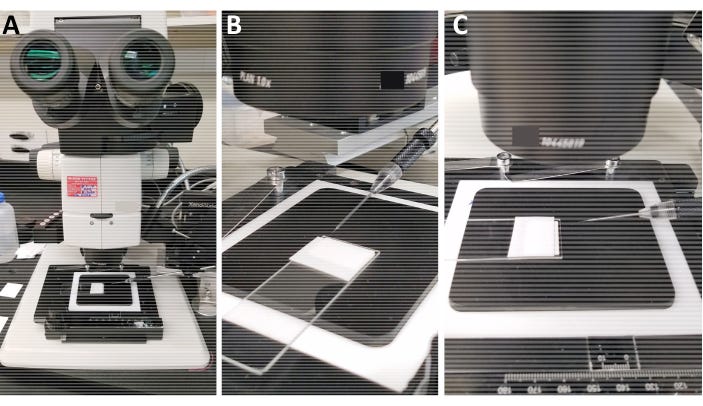
At the heart of modern gene drives is the CRISPR/Cas9 system. The commonly cited (current) version of gene drive constructs has two key parts: Cas9, which is the "scissors" that cuts DNA, and guide RNA (gRNA), which is the "targeting system" that tells Cas9 exactly where to cut. Think of it like a heat-seeking missile: the guide RNA seeks out a specific DNA sequence, and once it finds it, Cas9 moves in to make the cut. A promoter is used to endogenously express this construct, leading to cuts at desired sites throughout germ cells. WIth mosquitoes, we produce this construct in one plasmid (Golden Gate works great for this, in my experience, since the constructs end up being 5+ components), then directly inject it into embryos. Though many embryos die, it works fairly well to get a starting parental population – my last experiment doing this resulted in 125 hatched larvae from 4125 injections (3.03% hatch rate). You can see the setup for how this is done in Figure 7a/b below.
Figure 7a. The setup for mosquito embryo microinjection involves lining up a few dozen mosquito eggs against a piece of wet paper on a slide and injecting them with ~3ul of dissolved plasmid (Protocol for those curious, and source for the image above). As you can see (or not), mosquito embryos are quite small, so this process involves a microscope to visualize the puncture+injection.
Figure 7b. This is what things look like under the microscope. The sharp bit you see at the top is the quartz needle used for the process (A nice little writeup if you want to learn more, and also the source of the image)
What makes this system so powerful for gene drives is its precision and programmability. Guide RNAs are designed to match a specific 20-letter sequence of DNA bases (A, T, C, and G). When the guide RNA finds its matching sequence in the mosquito's DNA, it binds to it like a zipper coming together. This binding creates a signal that activates Cas9, which then makes a clean cut through both strands of the DNA double helix. In gene drives, we typically target sequences that are highly conserved, which generally means things that are critical for mosquito function and survival. This is crucial because even a single letter change in the target sequence can prevent the guide RNA from binding, rendering the gene drive ineffective, which brings us to our next section.
Guide RNAs rule everything around me
We understand now that gRNAs are a key component to gene drives, because they act as the homing system to our molecular cutlery. Making gene drives work requires a specific type of DNA repair to happen when damage happens. When we use CRISPR/Cas9 to edit genes in organisms, there are two potential events that can occur: non-homologous end-joining (bad) or template-directed repair (good). When Cas9 cuts DNA, it creates what's called a double-stranded break - imagine cutting both strands of the DNA double helix with molecular scissors. The cell recognizes this as damage and kicks into repair mode. The simple but messy solution is non-homologous end-joining (NHEJ). Think of it as the cell's emergency repair system - it grabs the broken ends and sticks them back together, often introducing small errors in the process. It's like trying to fix a broken rope by quickly tying the ends together - it works, but it's not pretty. For gene drives, NHEJ is bad because it can create mutations that make the target site unrecognizable to the gRNA, effectively immunizing that chromosome against future cutting attempts. Any future attempts at editing are thereby disrupted, since the guide no longer has a landing site, breaking the drive’s function. Because gene drives are designed for deployment in germline editing, these drive-breaking alleles propagate through a population should they arise – quite the opposite of the intended effect of gene drives!
What we want instead is homology-directed repair (HDR), also called template-directed repair. The cell looks for a similar DNA sequence to use as a template - and conveniently, we've provided one on the chromosome carrying our gene drive when we inject a construct into the embryo through microinjection. When HDR kicks in, it copies our gene drive sequence into the cut site, creating a perfect duplicate. This is why gene drives can spread so effectively: they turn the cell's own repair machinery into a copying system. It’s roughly estimated that a cell undergoes NHEJ about 75% of the time there is a double-stranded break, with HDR capturing the rest of breaks that happen. It’s worth noting that there are specific time points where HDR becomes more common, with HDR taking a lead with cells between the G2 and S transition. Using a promoter to express Cas9 that functions during this G2/S time point is one way of biasing towards the more ideal repair mechanism, and it’s something that’s being actively tried with some success.
If we zoom out, more globally the issue with these gene drives is we can't fully control which repair pathway the cell chooses. Different organisms, cell types, and even life stages can favor one pathway over the other. This becomes particularly important in germline cells - the cells that will become eggs or sperm - because that's where the gene drive needs to work for successful inheritance. In mosquitoes, getting this balance right has proven to be one of the major technical hurdles in developing effective gene drives, though I’ll note that the team of Andrew Hammond, Andrea Crisanti and Tony Nolan are hard at work with smart mechanisms to improve HDR using germline restricted promoters.
Considering the importance of the guide to making this all work seamlessly, another issue arises when SNPs happen. The use of multiple gRNAs helps in making sure Cas9 is only cutting exactly where we want to, but carries the double edged sword of creating more opportunity for things going awry as the result of just a couple mutations. Genetic engineers have come up with two main strategies for avoiding this, though, both of which are very clever: 1) they insert drive constructs in regions that become lethal to developing embryos if mutated and 2) select these regions of high importance with the hope that SNPs are less common. I’m happy to report that at least in cage experiments, the field has seen an impressive ability to create drive-resistant – or even better, drive-deleting – alleles with robust engineering strategies.
Everything to this point suggests things have been going very well, a feat owed to the work of a bunch of incredible scientists doing really painful work rearing, editing, and breeding mosquitoes, often with their own blood sacrifice. But my skepticism is born out of something a little less grounded in facts, and more on vibes. Let me try to explain.
Evolution doesn’t like us messing with things
The below section is a little more hand-wavy, but it touches on why I believe – purely from vibe / intuition – that gene drives will be very difficult to make work at sufficient scale to matter, or at least compared to other things. Please understand this is really just my taste, developed through working on this problem for only a couple years, and also my experience that engineering complex systems is extremely difficult. Messing with biology is just really hard, and it often fights back.
Unlike Drosophila, mosquitoes are not a “solved” organism. Biologists use Drosophila to study many important features of genetics, aided by the fact that fly biology is well documented with many great tools developed for their study. Unfortunately, this doesn’t exist for mosquitoes. The field’s best database, Vectorbase, actually was under threat of going down earlier this year, but was saved by a group of non-profit funders and is still operational (at least as of writing).
This is a really, really important point. Some iterations of gene drives have been demonstrated to work in fruit flies, making use of a hundred years of intensive research effort, but mosquitoes unfortunately are not flies, and as such, remain shrouded in (some) mystery. Entomologists didn’t have a full Anopheles genome until 2014 – something available to anyone studying Drosophila melanogaster in 2000! More generally, this is emblematic of something I hope every reader will take away, which is that mosquitoes are very complicated and we do not fully understand them. What I mean by this is that many of the things we take for granted in other fields are not available to mosquito biologists, and though they do tremendous things with the tools available to them, there are limitations.
Take as an example, the question of insecticide resistance. An unfortunate consequence of permethrin and other insecticides for malaria control is that the organism has selected for individual mosquitoes that stay alive when challenged by these chemicals. This happens in many other cases, like bacteria outstripping antibiotics at a speed greater than we can produce more, but unlike bacteria, we actually didn’t quite understand this process until recently. As it turns out, the mating dance the mosquitoes do in mating swarms involve tapping/testing the cuticle thickness of males to determine fitness, which, it turns out, is the exact feature that makes mosquitoes resistant to insecticides. I’m sure some readers will think of some way to engineer around this, but it seems likely some other obstacle will become a hurdle. See the below for what I’m talking about.
Being infected with malaria parasites has no fitness cost to the female mosquito. The major challenge to our ability to do anything in biology often comes down to how much nature resists us. In this case, there are challenges presented by the fact that malaria-infected mosquitoes do not have any fecundity deficits. Some labs have even reported that malaria infection actually drives mosquitoes to oviposit earlier (mosquito version of giving birth).
Evolution is complicated, and the mechanisms that have gotten enduring species to this point are robust, with a significant amount of redundancy to capture the best and most important features. In the case described above, mosquitoes are seeing some types of fecundity / fertility benefits from being infected with Plasmodium parasites. In another case we see that the most insecticide resistant mosquitoes – which we established earlier are the result of thicker, fitter thorax/abdomen selected for in mating – actually promotes Plasmodium infection in the mosquito. What I’m getting at with these obscure references to basic mosquito biology is that things are complicated, and what may seem obvious with rational approaches can cause us issues that are not clear until much later.
Nations deserve some amount of independence here. Now, I understand this isn’t a scientific or engineering point, but I want to cover it regardless. A common argument I hear from people (or read in the NYTimes) is that gene drives work, and we just need to start testing them in the wild to demonstrate that. It may be true that drives work in cage experiments quite well, but we’re generally making this argument with the hope of testing these in someone else’s backyard. Gene drives are mostly discussed for release in malaria endemic areas, not managing West Nile or EEE outside of Boston, or Zika in Florida. I believe in autonomy, and think giving independent, albeit resource-limited nations a chance to dictate their own fate is worthwhile and important, even if we strongly believe our technology will work. It’s ultimately them who will have to live with the consequences of affected ecological niches, or pay a bunch of money for continuous releases if evolutionary stable drives don’t pan out. I mention this in the spirit of self-governance, but I’m sure there are nations that want to try this, and to them I say go for it — the global community would be in your debt if you elected to do so.
Some last points
I truly hope the scientific community can solve the malaria problem, and I firmly believe it will happen. What I’m hoping to impart on the readers here is not a pessimism for what we can accomplish with biological engineering, but rather offer my opinion about why things haven’t worked out to this point, and more importantly, why it’s worth exploring other options to conquering these pests.
To that end, I’m happy to say that there are other very clever things biologists have come up with. I will list just a couple of them here, and why I’m hopeful.
Infecting malaria-carrying mosquitoes with Wolbachia bacteria. It turns out that there’s an incredible symbiotic relationship that exists between certain types of non-malaria carrying mosquitoes, Culex species, and an obligate intracellular bacteria Wolbachia. When infected, mosquitoes become incapable of carrying viruses that cause Dengue, West Nile and Zika, and more, as recently discovered, malaria. The genes involved in this process, cifA and cifB, when transferred to mosquitoes results in conditional sterility – a potential other tool for malaria control. Some groups are working to transfer these Culex-infecting Wolbachia bacteria to malaria-carrying Anopheles mosquitoes with the hopes of breaking the capability of Anophelines to be disease vectors. This seems exciting, mostly because this is something that occurs in nature, and more importantly, is easily reversible with antibiotic treatment if something goes wrong.
Though this is one bullet point, consider it two separate concepts – engineering mosquitoes to carry cifA/B, or infecting mosquitoes with Wolbachia. Oxitec is working on Wolbachia as an approach to genetically modifying mosquitoes sustainably, and are doing releases now.
Treating mosquito nets with human malaria drugs. My former colleagues at Harvard, namely Doug Paton (who runs his own lab at the University of Georgia), came up with the genius idea of soaking mosquito nets in the malaria drug atovoquone. It turns out this works very well at ridding the mosquito of the malaria parasite. Resistance is always a concern with these types of approaches, but it is incredible that this works at all – my expectation is that little tricks like this can add up to big gains, when combined with existing mosquito net and insecticide paint treatment in houses.
Functional malaria vaccines seem to be well underway. Technologies successfully deployed in the COVID era have great potential to unlock a functional malaria vaccine. There is exciting work coming out of both academic and industry groups, and there is reason to be hopeful.
There is a lot to be excited about when it comes to the future of malaria control coming in from many different directions. It is a terrible disease that has killed more people than any other infection on the planet, and of course people will always get excited about new approaches to handling difficult problems. Gene drives are exciting for scientists and laymen alike, because it’s an application of human ingenuity to solve the problems caused by forces outside of our control – something I very much believe in. But being chained to continuous releases is a considerable obstacle, and it’s worth considering whether this is better than existing alternatives, like more investment in nets, eliminating standing water in living areas, and development of vaccines. I just think ultimately there are other exciting approaches worth exploring, and would hate to see the enthusiasm to solve such a significant challenge dwindle due to the difficulty of deploying gene drives in the world.
Tl;dr
A generous donation from Perran Ross, PhD — a common practice in mosquito research.
Come on, can you please read the post? I didn’t blood feed thousands of mosquitoes from my own hand in a lab just so you can shortcut the process of learning from my suffering.
Acknowledgements
Thanks to Stephen Malina, Willy Chertman, and Effie Klimi for input on this piece, and to Merrick for presenting a well-reasoned counter-argument, which swayed me to be more optimistic.













Luckily, I didn't blood feed any mosquitos so you can feel free to shortcut the process of learning! Of course I also didn't do any of the research, so take my shallow dive with a grain of salt.
The problems boil down to this: Gene drives need perfect genetic editing to work across generations, but biology keeps throwing wrenches in the system. When cells repair DNA cuts, they usually pick the "quick and dirty" repair method (NHEJ) over the precise one we need (HDR) - we're talking 75% vs 25% odds. Plus, mosquitoes are wildly under-studied compared to lab favorites like fruit flies, so we keep running into surprise roadblocks with their biology. Think about bacteria developing antibiotic resistance, but more complex. To top it off, maintaining these modified populations requires constant releases of new mosquitoes, making it a resource sink rather than a one-and-done solution.